Introduction:
The management of classical Hodgkin lymphoma (cHL) with first line therapy continues to evolve resulting in some of the best outcomes in oncology. Around 20-30% of patients with cHL relapse and have to undergo salvage chemotherapy followed by autologous hematopoietic cell transplant (Auto HCT). There are no randomized trials comparing the most common regimens used in second line to date. With the introduction of newer agents in the treatment of cHL, it is essential to identify patients that might benefit from a different approach in first salvage setting. The aim of this study is to assess patterns of relapse and differences in outcomes based on salvage regimens.
Methods:
We retrospectively identified 193 consecutive patients with cHL treated in our academic medical center. Adult patients treated with ABVD (Adriamycin, Bleomycin, Vinblastine, Decarbazine) for first line were included. The three most common salvage regimens used in our institution included ICE (Ifosfamide, Carboplatin, Etoposide), ESHAP (Etoposide, Steroids, Ara-C, Cisplatin), or IGEV (ifosfamide, Gemcitabine, Vinorelbine). Patients were divided into two groups: those progressing during ABVD or relapsing within 1 year are considered (refractory) versus all other as (late-relapse). Response to salvage therapy and performance of Auto HCT were compared using Chi-square test. Univariate comparison of OS was conducted using Log-rank test.
Results:
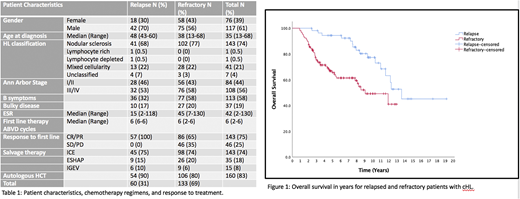
The majority of patients were refractory to front line therapy (69%). As expected, there was predominance of male gender (61%), with median age of 35 yrs (range 13-68 yrs). The distribution of baseline characteristics did not seem to be different between the two groups (Table 1). Refractory patients were more likely to have B symptoms and higher ESR on presentation (Table 1). Of the refractory patients, the majority had initial response to therapy then relapsed within 1 year (65%). On Univariate analysis, late-relapse patients had better overall survival compared to refractory disease (13.5 yrs vs 9 yrs respectively) (p<0.005) (Figure 1).
The majority of the patients in both groups received ICE as the primary regimen, followed by ESHAP and IGEV (Table 1). The CR rate was not statistically different among the three salvage regimens (ICE, ESHAP, IGEV as 37%, 29%, 53% respectively) (P=0.248). Late-relapses were more likely to obtain a complete response (CR) to salvage therapy (48% vs 32% respectively) (P=0.025). However, when CR rate stratified by salvage regimen, only refractory patients treated with ICE demonstrated statistically significant worse CR rate compared to late-relapse treated with ICE (32% vs 48% respectively) (P=0.047). The difference was not statistically different for ESHAP and IGEV.
While there was a difference in CR rate between relapsed and refractory patients, the difference was not observed in the rate of consolidative Auto HCT between the two groups.
On univariate analysis, Auto HCT was associated with improved median overall survival compared to no Auto HCT (11.3 yrs vs 1.6 yrs, respectively) (P<0.001).
Conclusions:
To our knowledge, this is the largest retrospective comparison study on the outcome of the most common salvage chemotherapy regimens in cHL. Our study highlights the unmet need early introduction for novel non chemotherapy-based interventions in refractory patients. It also shows the consistent CR rate in real world experience in comparison with clinical trials and persistent need for Auto HCT in the salvage setting in the current chemotherapy era.
Chavez:BeiGene:Speakers Bureau;Bayer:Consultancy;Merck:Research Funding;Morphosys:Consultancy, Speakers Bureau;AstraZeneca:Speakers Bureau;Celgene:Consultancy;Novartis:Consultancy;AbbVie:Consultancy;Genentech:Speakers Bureau;Epizyme:Speakers Bureau;Gilead:Consultancy;Karyopharm:Consultancy;Kite, a Gilead Company:Consultancy, Speakers Bureau;Verastem:Consultancy;Pfizer:Consultancy.Shah:Kite/Gilead, Jazz, Incyte:Research Funding;Moffitt Cancer Center:Current Employment;NCCN: Vice-Chair, Acute Lymphoblastic Leukemia Working Group:Membership on an entity's Board of Directors or advisory committees;Kite/Gilead, Celgene/Juno/BMS, Novartis, Pfizer, Amgen, Spectrum/Acrotech, Precision Biosciences, Beigene, AstraZeneca, Pharmacyclics/Jansen, Adaptive:Honoraria;Kite/Gilead, Precision Biosciences, Novartis, AstraZeneca:Other: TRAVEL, ACCOMMODATIONS, EXPENSES.Sokol:EUSA Pharma:Consultancy, Honoraria, Speakers Bureau;Kyowa/Kirin Inc.:Membership on an entity's Board of Directors or advisory committees;Kymera Therapeutics:Membership on an entity's Board of Directors or advisory committees.Pinilla Ibarz:Takeda:Consultancy, Speakers Bureau;AstraZeneca:Consultancy, Speakers Bureau;Abbvie:Consultancy, Speakers Bureau;Novartis:Consultancy;Sunesis Pharmaceuticals:Consultancy;TG Therapeutics:Consultancy;Sanofi:Consultancy;Pharmacyclics:Consultancy, Speakers Bureau;Janssen:Consultancy, Speakers Bureau.Bello:Celgene:Consultancy, Honoraria, Speakers Bureau;Seattle Genetics:Consultancy, Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal